
Electron beam crosslinking can enhance different polymer properties such as tensile strength, impact strength, high-heat properties, abrasion and chemical resistance, and environmental stress crack resistance. But not all polymers crosslink.
So how can you tell if your polymer is a good fit for e-beam crosslinking? One of the factors that help us know if a polymer is likely to crosslink or not is its glass transition temperature (Tg).
What is glass transition temperature?
Have you ever left something plastic outside during the winter, like, say, a potted plant you totally *meant* to bring inside before it froze solid? (Don’t worry, I won’t tell.) Chances are, when you do go to pick up the pot that next spring, it’s likely to crack. This is an example of the phenomenon known as glass transition. When a polymer is cooled below its glass transition temperature (known as its glassy state), it becomes hard and brittle, like (you guessed it!) glass. When a polymer is heated above its glass transition temperature (rubbery state), however, it becomes soft and flexible.
Below the transition temperature, the polymer chains are not able to move and rotate, which we have learned is not conducive to crosslinking. Above the glass transition temperature, however, the chains are able to move and crosslinking is more likely to occur.
(To be clear, glass transition is NOT the same thing as melting. Melting is a transition that occurs in crystalline polymers, when the polymer chains fall out of their crystalline structures and become disordered liquid. Glass transition occurs in amorphous polymers.
How is transition temperature related to crosslinking?
So how much of a relation is there really between crosslinking and the glass transition temperature?
Let’s look at the following figure, simplified from Radiation Processing of Polymer Materials and it Industrial Applications. The G value is a relation of the chemical yield resulting from radiation. G(X) is crosslinking and G(S) is chain scissioning. In the figure, we are looking at the results of irradiation conducted at room temperature and can see the lower the transition temperature, the higher the G(X) or amount of crosslinking. The results speak for themselves! 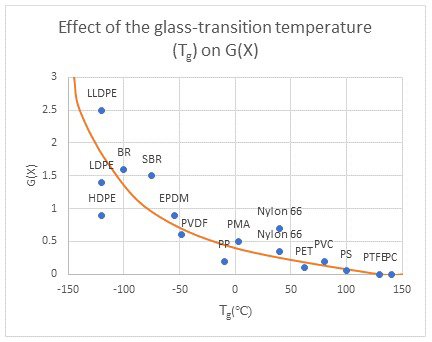
Of course, there are other factors involved…but that’s another entry.
Have a question about a specific polymer? Email us at ebeam@ebeamservices.com!




