E-BEAM Services Inc. is a industry leader in ISO/AAMI 11137 validation requirements for the sterilization of health care products using radiation.
Outside of the United States? We assure compliance with the EU Guidelines to Good Manufacturing Practice for Medicinal Products and the European Council Directive Concerning Medical Devices.
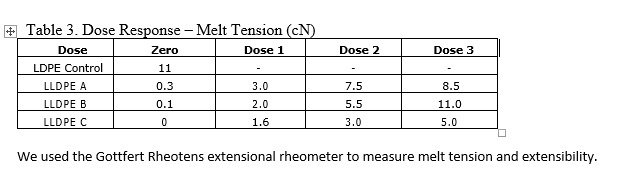
Materials Testing and Establishing Maximum Dose
 In order to determine if your product is compatible with e-beam sterilization, E-BEAM Services will set-up a simple experiment. This experiment includes exposing a number of samples to a range of doses to see the effects of the radiation on their product materials and the packaging. Not only will the experiment determine the product compatibility, it is also used to determine the maximum acceptable dose for the product.
In order to determine if your product is compatible with e-beam sterilization, E-BEAM Services will set-up a simple experiment. This experiment includes exposing a number of samples to a range of doses to see the effects of the radiation on their product materials and the packaging. Not only will the experiment determine the product compatibility, it is also used to determine the maximum acceptable dose for the product.
Call us for information: 513-933-0031
A typical experiment involves:
Exposing a number of product samples to a range of doses greater than anticipated during routine sterilization.
The number of samples depends on what you, the manufacturer, need in order to test the functionality of the product.
The doses range should be wide enough to determine the acceptable maximum dose.
As a manufacturer, you are required to establish and communicate to the sterilizer the sterilization dose that your product requires (minimum dose), and the maximum acceptable dose. The wider the range between the minimum and maximum doses, the greater the flexibility there is in the sterilization process – which leads to cost savings.
Establishing the maximum acceptable dose is a requirement of ANSI/AAMI/ISO 11137-1:2006, the Standard for sterilizing health care products using radiation. For guidance in testing and qualifying materials used in health care products that are sterilized with radiation, there is a technical report entitled AAMI TIR17:2008 which can be referenced

Product Dose Map and Subsequent Validation Runs
In electron beam processing, a dose map measures the distribution of dose, typically within a case of medical devices to be sterilized. You are looking to determine the maximum internal dose and the minimum internal dose, as it relates to a specific reference dose.
The process of a dose map involves placing a number of dosimeters within a case of product to identify the location of the minimum dose and the maximum dose. The number of dosimeters used, and location of the dosimeters depends on the uniformity of the packaging and the geometry of the devices inside. The dosimetry results show the distribution of dose throughout the case, and each data point is considered relative to a conveniently-monitored surface dose position.
These relationships are used to complete a processing plan or sterilization protocol. The product information required includes the minimum dose to achieve sterility or performance criteria and the maximum dose to maintain the intended efficacy and shelf life. Using the dose relationships and the minimum and maximum dose, we can calculate an acceptable surface dose range, which is used to monitor the routine production process.
As part of the validation requirements, a mini dose map is performed three subsequent times to verify the ratio findings of the initial dose map.
Once this work is completed, a formal dose map and validation report is prepared with specified processing parameters. This product will then be validated and as long as there are no changes to the product or packaging, it does not need to be repeated.
 Establishing Sterilization Dose and Sterilization Dose Audits
Establishing Sterilization Dose and Sterilization Dose Audits
The sterilization dose can be established by determining the actual bioburden of a product and establishing an average, or by using a 25 kGy dose and substantiating it. It is a requirement of ISO/AAMI 11137 to audit your sterilization dose before the initial production run, and then at intervals thereafter to ensure that it has not changed.
The sterilization dose audit is a product validation test designed to establish or confirm the sterilization dose by exposing test product to sub-cycle conditions. Dose audits are then sent to a microbiological lab to be evaluated.
While E-BEAM Services does not provide microbiological services such as bioburden testing or sterility testing, we can recommend reputable labs who provide these services.
