If crosslinking chemistry is like carpentry on a molecular level, then electron beam processing is like having a “secret weapon” in your tool belt. Instead of using time-consuming and complicated chemical crosslinking, let the electrons do the heavy lifting!
When a polymer is showered with high energy electrons, the electrons kick off hydrogens and create free radical sites along the polymer chains. These sites either terminate, resulting in chain scission, or link up with another chain, creating a “crosslink”. For an e-beam crosslinkable polymer, the overall result will be more crosslinks and therefore molecular crosslinking.
A polymer’s molecular structure will give some strong indications as to predicting whether it is more likely to undergo crosslinking, chain scissioning, or to resist radiation entirely.
Structures favoring Crosslinking chemistry
- Long chains of hydro-carbons without substitutions or pendant groups (polyethylene is a classic e-beam crosslinkable polymer)
- Saturated carbon rings
- Carbon-carbon double bonds
- Also note that trifunctional monomers as an additive can make an otherwise radiation-resistant polymer crosslinkable.
Aromatic rings, either in the main chain or as side groups, will cause the polymer to resist radiation, keeping the polymer from crosslinking or chain scissioning.
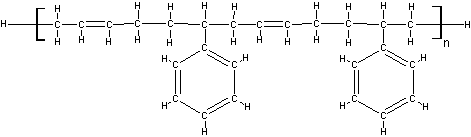
An Example Molecule
In a molecule with multiple group categories, the percentage of each helps determine if it will crosslink or scission. For example, the higher the hydrocarbon percentage, the more likely it is to crosslink.
Polyacrylate is a good example molecule, as the double bond in the backbone helps with crosslinking, but the benzene ring reduces crosslinking. These two groups cancel each other’s effects so on average it crosslinks like a plain hydrocarbon chain.

www.ebeamservices.com • Ohio (513) 933-0031 • New Jersey (609) 655-7460



