Crosslinking plastics, using e-beam processing, creates excellent property improvements. But just what is crosslinking? If you want the technical definition (which may make your eyes glaze over) it is:
With certain thermoplastics, the setting up of chemical links between molecular chains occurs. When extensive, crosslinking makes an infusible super molecule of all the chains. The phenomenon of crosslinking involves primary bonds between polymer chains creating a three-dimensional network.
The greater the degree of crosslinking, the greater the rigidity of the material, the less soluble, and less it responds to remelting. (Rosato’s Plastics Encyclopedia and Dictionary).
In short, ionizing radiation produced by the beam breaks bonds within molecules; when the bonds reform in a beneficial way, we get “crosslinking.”(In chain scissioning, the bonds remain broken; but let’s leave that topic for another day…).
In polyethylene, a bond with a hydrogen atom is broken, allowing the carbon backbone of the molecule to join with another carbon backbone of an adjacent molecule. The final result is an increase in the molecular weight of the polymer, imparting beneficial properties such as better abrasion resistance, less permeability, higher tensile strength, and higher operating temperatures.
A simple way of demonstrating the effects of crosslinking is to place one irradiated sample of polyethylene tubing and one un-irradiated sample in an oven, as seen here:
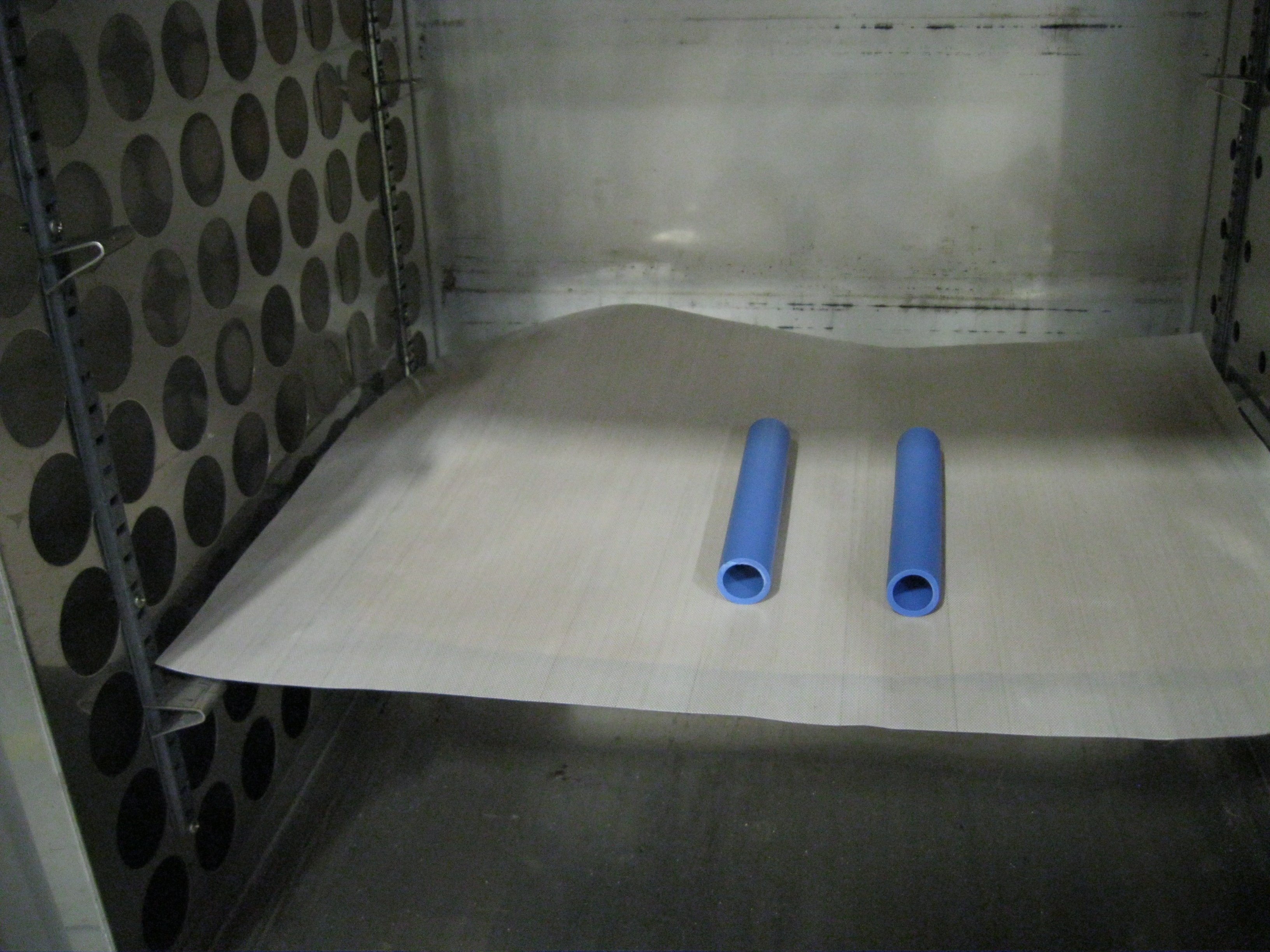
After 30 minutes at 200°C, these are the results:

Can you guess which sample has been irradiated? The sample on the left clearly has a higher operating temperature, due to crosslinking that occurred during e-beam processing. The sample on the right was not so lucky.
Have more crosslinking questions? Post them here, or give us a call!

www.ebeamservices.com • Ohio (513) 933-0031 • New Jersey (609) 655-7460